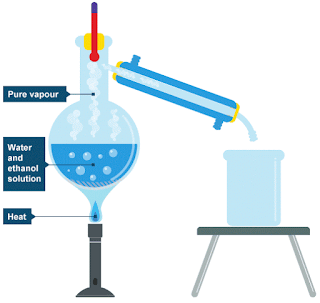
Chemicalformulas - Distillation is a commonly used method for purifying liquids and separating mixtures of liquids into their individual components. Familiar examples include the distillation of crude fermentation
broths into alcoholic spirits such as gin and vodka, and the fractionation of crude oil into useful
products such as gasoline and heating oil. In the organic lab, distillation is used for purifying
solvents and liquid reaction products.
The atmospheric distillation of petroleum products using a laboratory batch distillation unit is to determine quantitatively the boiling range characteristics of such products as light and middle distillates automotive spark-ignition engine fuels. Automotive spark-ignition engine fuels containing up to 10 % ethanol, aviation gasolines, aviation turbine fuels, 1-D and 2-D diesel fuels. Biodiesel blends up to 20 %, marine fuels, special petroleum spirits, naphthas, white spirits, kerosines, and Grades 1 and 2 burner fuels.
The basic test method of determining the boiling range of a petroleum product by performing a simple batch distillation has been in use as long as the petroleum industry has existed. It is one of the oldest test methods under the jurisdiction of ASTM Committee D02, dating from the time when it was still referred to as the Engler distillation. Since the test method has been in use for such an extended period, a tremendous number of historical data bases exist for estimating end-use sensitivity on products and processes.
The distillation (volatility) characteristics of hydrocarbons have an important effect on their safety and performance, especially in the case of fuels and solvents. The boiling range gives information on the composition, the properties, and the behavior of the fuel during storage and use. Volatility is the major determinant of the tendency of a hydrocarbon mixture to produce potentially explosive vapors.
Volatility, as it affects rate of evaporation, is an important factor in the application of many solvents, particularly those used in paints.
The distillation characteristics are critically important for both automotive and aviation gasolines, affecting starting, warm-up, and tendency to vapor lock at high operating temperature or at high altitude, or both. The presence of high boiling point components in these and other fuels can significantly affect the degree of formation of solid combustion deposits.
Distillation limits are often included in petroleum product specifications, in commercial contract agreements, process refinery/control applications, and for compliance to regulatory rules.
Tag : vacuum distillation, distillation column, fractional distillation, steam distillation, distilled water, azeotropic distillation, distilling, distilled, what is distillation, distillation apparatus, molecular distillation, crude oil distillation, alcohol distillation, simple distillation, distillation process, distillation tower, distillate, fractional distillation of crude oil, how to make distilled water, filtration, reactive distillation, distilation, membrane distillation, distill, what is distilled water, extractive distillation, how to distill water, water distiller, cryogenic distillation, distillates, distillation unit,

The large and complex petroleum industry is essential for the economy nowadays, it is difficult for a country to run smoothly without petroleum. Petroleum is the raw material that will ultimately provide power for our vehicles, roads paving, Petroleum testing
ReplyDeleteThanks for the blog loaded with so many information. Stopping by your blog helped me to get what I was looking for. Oil and gas training
ReplyDelete